A Non-invasive Method To Vaginal Tightening: Rafael J Perez, Md, Facog: Urogynecologist
Non Medical Vaginal Tightening Up Sexual Dysfunction Near Me
Dependable data are needed on treatments marketed to women as genital restoration. After getting therapy for bust cancer cells, more than 70% of ladies report sex-related health and wellness problems. Sexual wellness adjustments can additionally occur with virtually any kind of type of cancer therapy. In 2022, greater than 8.8 million females will be returning to their oncologists for monitoring, their gynecologists for annual tests, and their health care physicians for checkups.
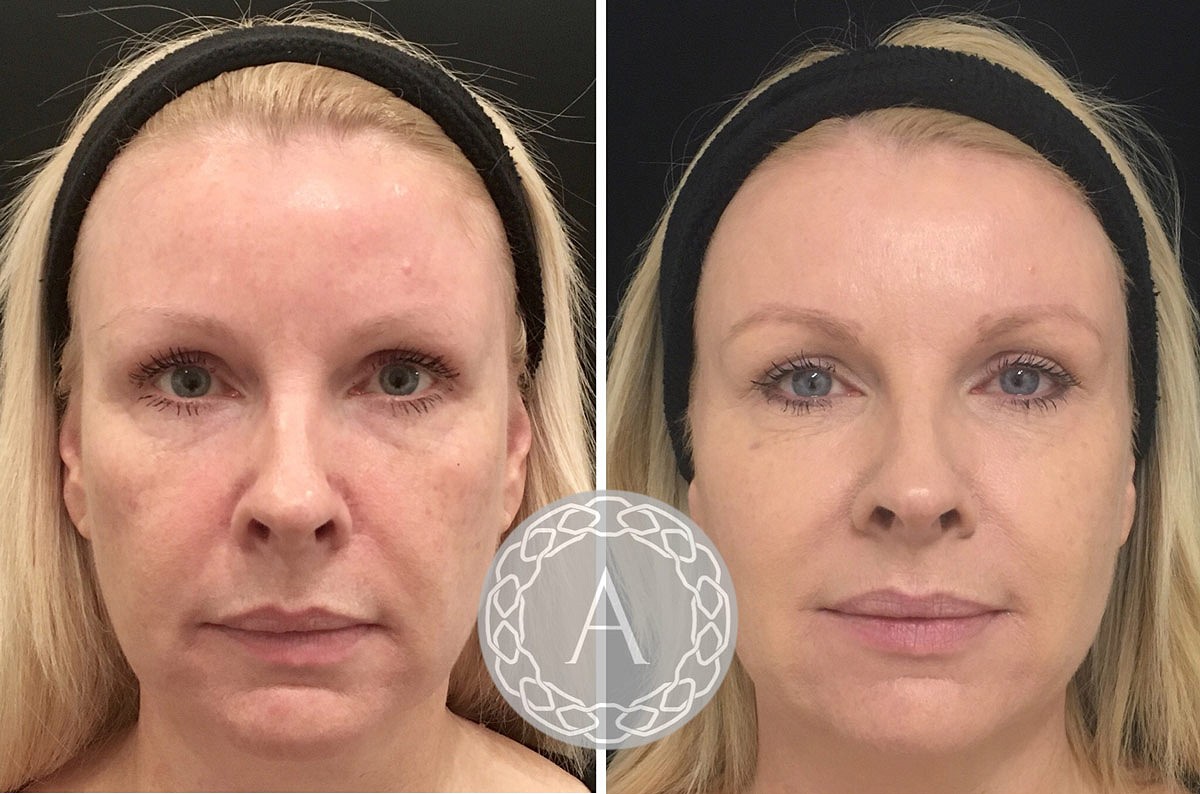
Revitalize & Renew: Non-surgical Genital Remediation
Your doctor will certainly provide you post-operative guidelines, including a checklist of limited tasks (like sex). FemiLift is a brand-new and ingenious approach to tightening up and enhancing the genital walls, leading to a smaller sized, tighter vaginal opening. FemiLift uses gold basic laser innovation to achieve these outcomes; this modern technology is scientifically risk-free and authorized by the FDA. The details offered on this website is provided for educational objectives only. This information is not intended to replace a medical consultation where a medical professional's judgment might recommend you about particular conditions, conditions and or therapy choices. We really hope the information will be useful for you to come to be extra informed about your healthcare decisions.
Vaginal Renewal And Cosmetic Genital Surgical Treatment
- Patients had significantly greater VHI ratings and substantial enhancements in dry skin, burning, itching, and dyspareunia.
- People frequently experience enhanced convenience and lowered symptoms of problems like urinary system incontinence.
- There are an absence of data with incongruity of outcome metrics making it hard to contrast gadgets and technology types.
- Vaginal distribution might lead to pelvic body organ fascia and tendon leisure, [3] muscular tissue damages along with vaginal leisure.
- Caution still remains nonetheless concerning their safety following a longer period of time after their usage.
- Nonsurgical vulvovaginal renewal (NVR) is a choice for women wanting reconstruction of youthful appearance and function of their genitalia.
The length of time that the results last can depend upon a range of elements, such as the individual's age, general health, and lifestyle routines. Likewise referred to as the G-Spot [Shot], this non-surgical treatment intends to improve sexual pleasure by infusing hyaluronic acid right into the G-spot location. Perineoplasty focuses on repairing and tightening the perineum, which is the area in between the genital opening and the rectum. This procedure is carried out to reconstruct the hymen, commonly for social or personal reasons. We frequently have occasions that people can be invited to, extending our message to all women.
Other nonhormonal therapy choices consist of PRP and lipofilling or fillers. Operation might last longer, but there is no warranty that the results will last permanently. Rapidly dealing with any type of interest in a healthcare provider can mitigate potential issues and guarantee a smooth recovery process. While it is possible to do ThermiVA while on your duration, it's best to wait until your period mores than before your ThermiVA therapy. A lot of insurer think about ThermiVA ® an elective treatment, and will not cover the expense of therapy.
The first 3 individuals in the study got 60 J/cm2 and since there were no unfavorable events, the next three patients received 75 J/cm2. Because no damaging events took place, the adhering to 18 clients obtained 90 J/cm2 with therapy times of thirty minutes. The writers theorized these power setting from ovine research studies with the device.21 Around 20 cm2 of the vaginal canal was treated with the therapy gadget put on the vaginal introitus from 1 to 11 o'clock settings while avoiding treatment to the urethra. Patients went through pelvic assessments at 1 and 3 months posttreatment and had completed Echogenicity the changed women sex-related feature index (mv-FSFI) and FSDS at 6 months.In this research, a temperature level regulated dual-mode (monopolar and bipolar) RF was put on individuals with genital laxity, and its efficiency and safety and security were examined to establish the therapy contentment. A minimal variety of scientific research studies have considered RF gadgets in NVR (Table 3). Millheiser et al checked out using Viveve in premenopausal ladies with self-diagnosed VL utilizing the VLQ.13 Clients had a testing physical and pelvic examination before therapy.
The writers discuss this constraint is since there is no present typical measuring device for such changes in the genital wall surface. In addition, the writers recognize the opportunity that the end results were a result of a placebo effect and recommended additionally comparison studies with a sham group. In July of 2018, the FDA released a warning versus using energy-based devices including laser and RF to perform genital rejuvenation procedures because of the truth that the security and efficacy of the gadgets had actually not been developed. The FDA stated that the gadgets had not been cleared for the treatment of GSM, SUI, and sex-related disorder or for aesthetic functions. This declaration was conjured up for concern that the energy-based devices might cause significant negative occasions including burns, scarring, dyspareunia, and chronic pain [65 •] ACOG released a position specifying that though the energy-based tools show potential energy they believed there wanted evidence for the efficacy and lasting safety and security of these treatments [66]